Acute Septic Arthritis is also known as infectious arthritis and is usually caused by bacteria. Notably, it can also be caused by a virus or fungus. Whereas, the condition is an inflammation of a joint that’s caused by infection.
Typically, acute septic arthritis affects one large joint in the body, such as the knee or hip. Less frequently, septic arthritis can affect multiple joints. With this in mind, allow the jmexclusives team to take you through the basics of Acute Septic Arthritis.
How does Acute Septic Arthritis occur?
In the first place, acute septic arthritis may develop as a result of hematogenous seeding, direct introduction, or extension from a contiguous focus of infection. Not to mention, the pathogenesis of acute septic arthritis is multifactorial. And depends on the interaction of the host immune response. In addition to the adherence factors, toxins, and immune avoidance strategies of the invading pathogen.
As a matter of fact, Neisseria gonorrhoeae and Staphylococcus aureus are used in discussing the host-pathogen. Especially, interaction in the pathogenesis of acute septic arthritis. While diagnosis rests on the;
- isolation of the bacterial species from synovial fluid samples,
- patient history,
- clinical presentation,
- laboratory findings,
- and imaging studies are also important.
Who’s at Risk for Septic Arthritis?
Young children and elderly adults are most likely to develop septic arthritis. People with open wounds are also at a higher risk for septic arthritis. In addition, people with a weakened immune system and those with pre-existing conditions such as cancer, diabetes, intravenous drug abuse, and immune deficiency disorders have a higher risk of septic arthritis.
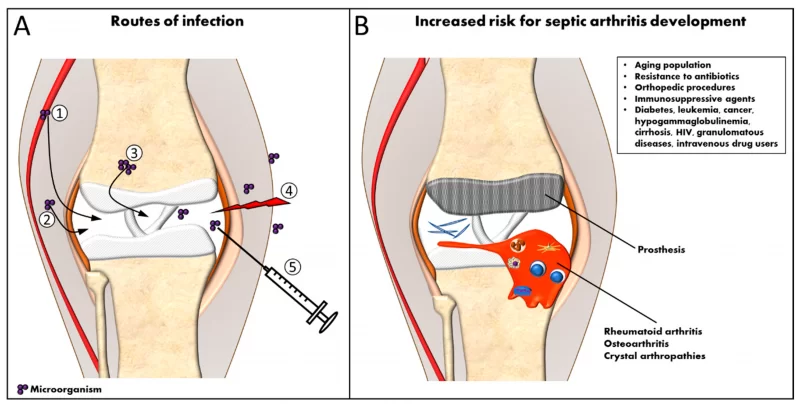
In addition, previously damaged joints have an increased likelihood of becoming infected.
What Are the Symptoms of Septic Arthritis?
Symptoms of septic arthritis usually come on rapidly with intense pain, joint swelling, and fever. Septic arthritis symptoms may include:
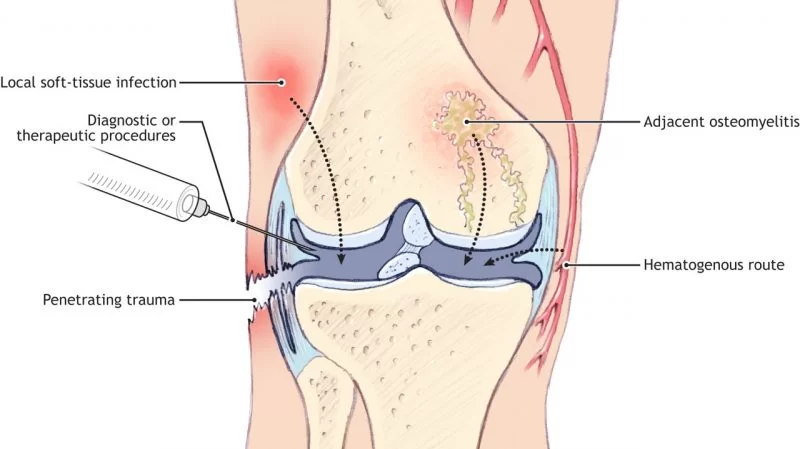
Sources of Acute Septic Infection
Acute nongonococcal septic arthritis is a medical emergency that can lead to significant morbidity and mortality.
Therefore, prompt recognition, rapid and aggressive antimicrobial therapy, and surgical treatment are critical to ensuring a good prognosis. Even with prompt diagnosis and treatment, high mortality and morbidity rates still occur.
Hematogenous seeding
Surprisingly, most septic joints develop as a result of hematogenous seeding of the vascular synovial membrane. Mainly, due to a bacteremic episode. Although a rare cause, acute septic arthritis may also occur as a result of joint aspiration. Or local corticosteroid joint injection.
Secondary Trauma
In addition, bacterial arthritis may arise secondary to penetrating trauma (such as human or animal bite or nail puncture). Equally too, after trauma to a joint without an obvious break in the skin. The direct introduction of bacteria during joint surgery has increasingly been a source of bacterial arthritis.
Particularly in association with knee and hip arthroplasties. When a bone infection breaks through the outer cortex and into the intracapsular region, a joint infection may also result. Especially in children. In infants, small capillaries cross the epiphyseal growth plate and permit extension of infection into the epiphysis and joint space.
Osteomyelitis infection
On one hand, in children older than 1 year, osteomyelitis infection presumably starts in the metaphyseal sinusoidal veins. And is usually contained by the growth plate. Whereby, the joint is spared unless the metaphysis is intracapsular.
On the other hand, the infection spreads laterally, where it breaks through the cortex. Lifting the loose periosteum to form a subperiosteal abscess. In adults, the growth plate has resorbed and the infection may again extend to the joint spaces.
Microbiology Association of Acute Septic Arthritis
Virtually every bacterial organism has been reported to cause septic arthritis. The microorganisms responsible for bacterial arthritis are largely dependent on host factors. Such as;
1. Staphylococcus aureus
Firstly, the most common etiological agent of all septic arthritis cases in Europe and all nongonococcal cases in the United States is Staphylococcus aureus. Whereas, the representation of S. aureus is more pronounced in patients with either rheumatoid arthritis or diabetes.
After S. aureus, Streptococcus spp. are the next most commonly isolated bacteria from adult patients with septic arthritis. While one study had a high representation of Streptococcus pneumoniae, Streptococcus pyogenes is usually the most common streptococcal isolate. Often, associated with autoimmune diseases, chronic skin infections, and trauma (94, 113, 141, 153).
2. Gram-negative bacilli
Groups B, G, C, and F, in order of decreasing preponderance, are also isolated. Especially in patients with;
- immunocompromise,
- diabetes mellitus,
- malignancy,
- and severe genitourinary
- or gastrointestinal infections (94, 113, 141, 153).
Gram-negative bacilli account for approximately 10 to 20% of cases (34, 94, 113, 141, 153, 183). In that case, patients with a history of;
- intravenous drug abuse,
- extremes of age,
- or immunocompromise display a higher prevalence of infection by gram-negative organisms.
Chiefly, the most common gram-negative organisms are Pseudomonas aeruginosa and Escherichia coli. As can be seen, anaerobes are also isolated in a small percentage of cases. Usually in diabetic patients and patients with prosthetic joints. Approximately 10% of patients with nongonococcal septic arthritis have polymicrobial infections.
3. Haemophilus influenzae
Historically, Haemophilus influenzae, S. aureus, and group A streptococci were the most common causes of infectious arthritis in children younger than 2 years. However, the overall incidence of H. influenzae as a cause of septic arthritis is decreasing because of the H. influenzae type b (Hib) vaccine now given to children.
A recent study of 165 cases of acute hematogenous osteomyelitis or septic arthritis treated in the years before and after the advent of the Hib vaccine demonstrated that musculoskeletal infections due to this bacterial species were reduced to nearly nonexistent levels.
Therefore, the coverage of H. influenzae as part of the empiric antibiotic coverage may no longer be needed. In general, for the management of acute septic arthritis in Hib-vaccinated children. While H. influenzae has lost its predominance.
4. Stomatitis
Clinical data suggest that the organism may gain access to the bloodstream in the course of an upper respiratory infection or stomatitis. In children older than 2 years, S. aureus, streptococci, H. influenzae, and N. gonorrhea have usually been isolated. Although H. influenzae may have also lost its predominance in patients in this age group.
5. Acute Septic Arthritis Microbiological Associations
Microbiological associations exist with concomitant disease states. Acute Septic Arthritis following cases of infectious diarrhea may be caused by;
- Shigella spp.,
- Salmonella spp.,
- Campylobacter spp.,
- or Yersinia spp.
However, these cases may reflect a form of reactive arthritis. A rare form of migrating polyarthritis may be caused by Streptobacillus moniliformis.
In human immunodeficiency virus (HIV)-infected patients, S. aureus continues to be the most common isolate (approximately 30%).
Pathogenic Acute Septic Arthritis
The pathogenesis of acute septic arthritis is multifactorial. And whereby, depends on the interaction of the host immune response and the invading pathogen.
By taking into account the steps of bacterial colonization, infection, and induction of the host inflammatory response, one may gain a greater understanding of this joint disease.
Nongonococcal Arthritis
Since S. aureus has been extensively studied with regard to its role in septic arthritis and causes the majority of cases in most nations. And the majority of nongonococcal cases in the United States.
Joint Colonization and Bacterial Adherence
The synovial membrane has no limiting basement plate under the well-vascularized synovium. Therefore, this allows easy hematogenous entry of bacteria. As mentioned above, bacteria may also gain entry into the joint by direct introduction or extension from a contiguous site of infection.
Once bacteria are seeded within the closed joint space, the low fluid shear conditions enable bacterial adherence and infection. Colonization may also be resultant especially in cases where the joint has undergone recent injury. In this environment, the production of host-derived extracellular matrix proteins that aid in joint healing.
For instance, fibronectin may promote bacterial attachment and progression to infection. The virulence and tropism of the microorganisms, combined with the resistance or susceptibility of the synovium to microbial invasion, are major determinants of joint infection.
S. aureus, Streptococcus spp., and N. gonorrhoeae are examples of bacteria that have a high degree of selectivity for the synovium, probably related to adherence characteristics and toxin production. Aerobic gram-negative bacilli such as Escherichia coli rarely infect the synovium except in the presence of an underlying and compromising condition.
The virulence of the organism once inside the joint varies. In rabbits, intra-articular injection of 10 5 S. aureus organisms into the knee joint resulted in major joint destruction but identical injections of N. gonorrhoeae or S. epidermidis caused no joint inflammation.
Joint Infection and the Host Immune Response
Once colonized, bacteria are able to rapidly proliferate and activate an acute inflammatory response. Initially, host inflammatory cytokines, including interleukin 1-β (IL-1β) and interleukin 6 (IL-6), are released into the joint fluid by synovial cells.
These cytokines activate the release of acute-phase proteins (e.g., C-reactive protein) from the liver that binds to the bacterial cells and thereby promote opsonization and activation of the complement system.
In addition, there is an accompanying influx of host inflammatory cells into the synovial membrane early in the infection. Phagocytosis of the bacteria by macrophages, synoviocytes, and polymorphonuclear cells occurs and is associated with the release of other inflammatory cytokines.
That includes tumor necrosis factor-alpha (TNF-α), IL-8, and granulocyte-macrophage-colony-stimulating factor. In addition to increasing the levels of IL-1β and IL-6, which are already present.
Acute Septic Arthritis Joint Damage
Under most circumstances, the host is able to mount a protective inflammatory response that contains the invading pathogen and resolves the infection. However, when the infection is not quickly cleared by the host, the potent activation of the immune response with the associated high levels of cytokines and reactive oxygen species leads to joint destruction.
High cytokine concentrations increase the release of host matrix metalloproteinases (including stromelysin and gelatinase A/B) and other collagen-degrading enzymes. When monoclonal antibodies or steroids attenuate these cytokines, cartilage degradation is minimized.
The joint is further damaged by the release of lysosomal enzymes and bacterial toxins. Host proteoglycans are initially degraded, and this is followed by collagen degradation. In fact, the polymorphonuclear response with subsequent release of these proteolytic enzymes can lead to permanent destruction of intra-articular cartilage and subchondral bone loss in as little as 3 days.
Metalloproteinases and the antigen-induced inflammatory response may persist and continue to damage the joint architecture even after the infection has been cleared. The infectious process induces a joint effusion that increases intra-articular pressure, mechanically impeding blood and nutrient supply to the joint. Thus, increased pressure destroys the synovium and cartilage.
Because of the proximity of the epiphyseal growth plate to the joint, direct extension of a joint infection to any of the articulating bones may lead to decreased bone growth in infants and children. In addition, the infection can spread to surrounding soft tissue, form sinus tracts, and disrupt ligaments and tendons in the untreated state.
Bacterial Products and their Pathogenic Role
While bacterial attachment proteins promote colonization and initiate the infectious process, a number of bacterial products activate the host immune response and increase tissue damage in cases of septic arthritis.
During acute septic arthritis, the innate immune system responds to the presence of the peptidoglycan wall (via N-formylmethionine proteins and teichoic acids) of S. aureus to produce proinflammatory cytokines (such as IL-1β, IL-6, and TNF-α) and C-reactive protein. Bacterial DNA (specifically unmethylated CpG motifs) also elicits an intense inflammatory response.
Bacterial Clearance vs Joint Damage
The interaction of the bacteria and host is of utmost importance in the initiation and prolongation of infection and cartilage damage. There is a subtle balance between an effective immune response to eliminate the infecting organism from the host and the overactivation of this response that causes the majority of infection-related joint destruction.
Gonococcal Arthritis
Gonococcal arthritis occurs in approximately 42 to 85% of patients. With disseminated gonococcal infection (DGI) and begins with localized mucosal infection. DGI-producing strains are unusually sensitive to in vitro killing by penicillin G. And possess unique nutritional requirements for arginine, hypoxanthine, and uracil. N. gonorrhoeae possesses a number of virulence factors.
It is the combined effects of these factors, their phase and antigenic variation, and properties of the host immune response that enable this pathogen to persist and allow the localized infection to become DGI. Notably, N. gonorrhoeae possesses a number of cell surface structures that have been implicated in virulence.
Protein I
Protein I is the main protein on the outer membrane. Strains that are able to cause disseminated infection in hosts with a normal immune system display serum resistance. Protein IA enables stable serum resistance by binding the host factor H.
This bacterially- bound host factor efficiently inactivates C3b (a central factor in both the classical and alternative complement cascades) into iC3b. Thereby reducing the efficacy of the host complement system.
This porin may also be responsible for the prevention of phagolysosomal fusion in polymorphonuclear leukocytes and a reduced oxidative burst, thereby enabling survival within these cells. This protein is thought to cooperate in the more intimate attachment following initial pilus interaction.
In addition, protein II is able to attach to the lipooligosaccharide (LOS) of other N. gonorrhoeae organisms. Thereby enabling the cells to bind to one other and form microcolonies. These microcolonies may also aid in the initiation of mucosal surface attachment.
Protein II is capable of avoiding clearance by the host immune system by phase and antigenic variation. Phase variation occurs through slipped-strand synthesis that produces a frameshift mutation and produces a prematurely terminated form of the protein.
Protein III
In addition, multiple variants of the protein II gene exist, and therefore the antigenic character of protein II can be changed by homologous recombination between these variants. While this protein is important for mucosal infections, most isolates from patients with DGI are missing protein II from their outer membrane and grow to form transparent colonies.
Protein III is another porin that is prevalent on the bacterial surface. The antibodies directed against protein III are not bactericidal, and they sterically inhibit antibody binding to protein I and unsialylated LOS that would probably result in bactericidal action. Therefore, the generation of these blocking antibodies may prevent serum bactericidal action.
Lipopolysaccharide
LOS is like the lipopolysaccharide of other gram-negative bacteria. Except that its carbohydrate portion does not have the complex structure of the repeating O side chain. This covalent attachment coats the bacterial cell in host proteins and avoids complement activation.
In addition, N. gonorrhoeae also produces an immunoglobulin A protease that may aid in colonization. However, the relevance of this potential virulence factor in gonococcal pathogenesis needs further study.
Host Factors for Acute Septic Arthritis
The host may contain a gonococcal infection through the action of the innate immune response, with particular dependence on the complement system. This system is largely responsible for attracting polymorphonuclear leukocytes and the resulting cascade of inflammatory cytokines and chemokines.
However, during periods surrounding early pregnancy, puerperium, and menstruation, the accompanying alterations in vaginal pH, cervical mucus, and genital flora and the endometrial exposure of submucosal vessels may predispose the female patient to N. gonorrhoeae invasion and DGI.
As mentioned above, defects in the complement and/or reticuloendothelial systems may also inhibit the host’s ability to contain the gonococcal infection.
Joint Risk Factors on Acute Septic Arthritis
Besides the obvious risk of acute septic arthritis associated with age older than 60 years and recent bacteremia, certain medical conditions predispose joints to nongonococcal infection.
Degenerative joint disease, rheumatoid arthritis, and corticosteroid therapy are the most common predisposing conditions. Specifically, patients with rheumatoid arthritis have an approximately 10-fold-higher incidence of septic arthritis than does the general population. In general, patients with;
- diabetes mellitus,
- leukemia,
- cirrhosis,
- granulomatous diseases,
- cancer,
- hypogammaglobulinemia,
- intravenous substance abuse,
- or renal disease
- and patients undergoing cytotoxic chemotherapy also have an increased incidence of septic arthritis.
Whereby, total joint arthroplasties are susceptible to intraoperative or hematogenous seeding and subsequent prosthetic joint infections.
While patients infected with HIV demonstrate a higher prevalence of musculoskeletal infections than does the general population (approximately 60 and 2 to 10 cases per 100,000 persons per year, respectively), it is unclear if this higher occurrence is due to the common septic arthritis risk factors due to intravenous drug abuse and multiple transfusions in this patient population.
Pathogen Risk Factors for Acute Septic Arthritis
In 0.5 to 3% of gonorrhea infections, the pathogen is able to gain access to the bloodstream from the primary mucosal site of infection and produce DGI. Risk factors include;
- infection with transparent,
- piliated N. gonorrhoeae strains capable of phase variation;
- diagnosis delay (especially in females due to the asymptomatic nature of the infection);
- complement system deficiency;
- systemic lupus erythematosus;
- menstruation,
- pregnancy, and puerperium;
- male homosexuality;
- urban residence;
- promiscuity;
- and low socioeconomic and educational status.
Females are four times as likely to develop DGI as males. This prevalence in women may be due to the asymptomatic nature of gonorrhea infections in women and the associated delay in diagnosis, thereby providing time for the bacteria to gain access to the bloodstream. In addition, many affected females are either pregnant or menstruating at the time of the infection.
Also, since the clearance of gonococcal infection depends on an effective complement-mediated immunity and a functional reticuloendothelial system, complement deficiencies and systemic lupus erythematosus are risk factors in this patient subset.
Diagnosis of Nongonococcal Arthritis
Nongonococcal septic arthritis is a medical emergency that can lead to serious sequelae and mortality. Therefore, prompt recognition and treatment are critical to ensuring a good prognosis. The classical presentation of acute nongonococcal septic arthritis includes the recent onset of fever, malaise, and local findings.
Especially of pain, warmth, swelling, and decreased range of motion in the involved joint. Moreover, a significant number of patients have a mild fever and may not demonstrate localized heat and erythema around the affected joint. The clinician should obtain a detailed history with special emphasis. Especially on determining the presence of any risk factors discussed above.
However, the diagnosis of infectious arthritis rests on the isolation of the pathogen(s) from the aspirated joint fluid. Most patients display elevated C-reactive protein levels and erythrocyte sedimentation rates. Synovial fluid analysis is also very important and usually reveals turbid, low-viscosity fluid with leukocyte counts usually in excess of 50,000/mm3.
However, nonbacterial inflammatory processes, such as acute crystalline joint disease or reactive arthritis, may have counts above this level while gonococcal and granulomatous arthritis may have counts below 50,000/mm3.
Other More Related Resources:
- Who does Osteoarthritis affect?
- Why is it Important to Have Healthy Gums?
- Eye Protection and Safety Measures You Should Know
- Psoriatic Arthritis Natural & Herbal Remedies
- Cancer Signs | Top 10 Early Symptoms You Should Never Ignore
Finally, I hope you enjoyed reading the above-revised guide on how to maintain Acute Septic Arthritis naturally. Therefore, help us share the word with other readers online. But, if you’ll have additional contributions, suggestions, and recommendations, please Contact Us. Or even, leave them in the comments box below this post.